mina.astron
Oct. 29, 2023, 3 min read,
Phytochemicals
Phytochemicals, natural products, secondary or specialized metabolites are synthesized by plants, microorganisms, marine algae, sponges, etc. for defending their producers against their predators including herbivores, carnivores, microbes and even to protect them against competing plants and abiotic threatens like free radicals. These compounds, depending on their biosynthetic pathways are classified in various groups like, terpenoids, alkaloids and phenolics.
Terpenoids
Terpenoids are one of the biggest class of phytochemicals with more than 35000 known chemical structures. The word is derived from turpentine, an oil from distillation of oleoresins of pine trees. Chemically, they have consisted of C5 isoprene (2-methyl-1,3-butadiene) unites that are connected from head to tail (Fig. 1). Depending to the number (n) of the isoprene units, terpenes can be classified into hemi- (n=1), mono- (n=2), sesqui-(n=3), di- (n=4), sester-(n=5) and tri-(n=6) terpenoids. Steroids with 6, but less than 30 carbon atoms, and carotenoids with n=8 isoprene units are modified forms of terpenoids and sometimes considered as primary metabolites. The mono- and sesquiterpenoids are present in the essential oils and have pleasant smells, while heaver terpenoids like di-and triterpenes are isolated from the solvent extracts of plants. Some of the terpenoids are used as medicines like paclitaxel and artemisinin; the anticancer and antimalaria drugs.
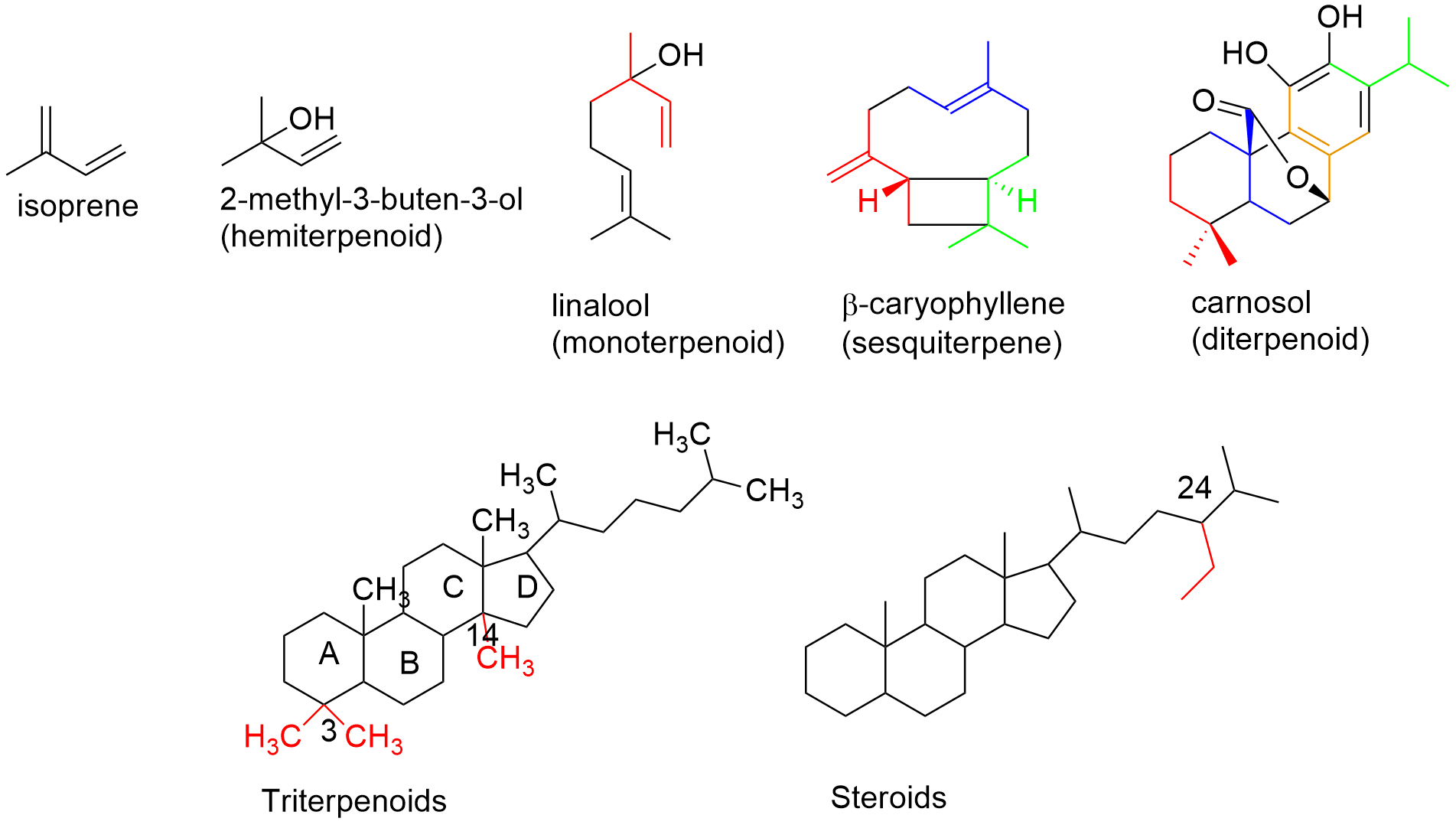
Fig.1. Chemical structures of different terpenoids; presenting isoprene units in colors.
Alkaloids
The second big class of natural products with more than 27000 phytochemicals is alkaloids. The term alkaloids derive from the compound’s alkaline or base like character. These compounds with having Nitrogen(s) atoms can be protonated which cause their solution to be basic. The origin of the N atom is from the alkaloid’s amino acids precursors. In addition to the true alkaloids originating from amino acids metabolisms, some other class of alkaloids take their N from trans amination reactions, but their carbon skeletons are belonged to terpenoids, steroids etc. They called pseudo alkaloids, like steroidal alkaloids. The major alkaloids depending on their biosynthetic pathways are grouped as pyridine, indole, quinoline, isoquinoline, etc. The biological activity of alkaloids is mostly depended on the degree of protonation of their nitrogen at physiological pH. Some of the most important medicinal alkaloids are morphine, caffeine, nicotine and colchicine (Fig. 2).
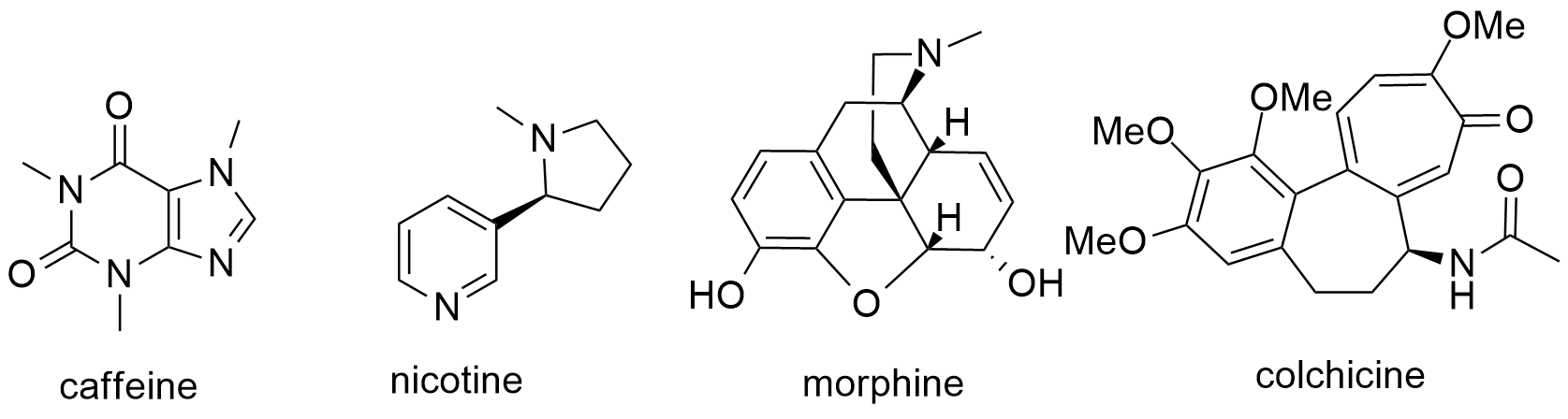
Fig. 2. The chemical structures of some medicinal alkaloids
Phenolics
The phenolic phytochemicals have at least one hydroxyl group on their aromatic ring in their molecules. Therefore, the phenolic phytochemicals can be obtained from each class of natural products that can produce phenol function. But the major phenolic compounds can be synthesized via shikimate or acetate pathways in vivo. The major phenolic are named as flavonoids, phenylpropanoids, cinnamic and benzoic acid derivatives, hydrolysable and condensed tannins, coumarins, and polycyclic phenolics (Fig. 3). They are widely distributed in fruits and have diverse ecological and biological roles in plants such as feeding deterrent, antioxidant, anti-inflammatory, anticancer and antimicrobial activities.
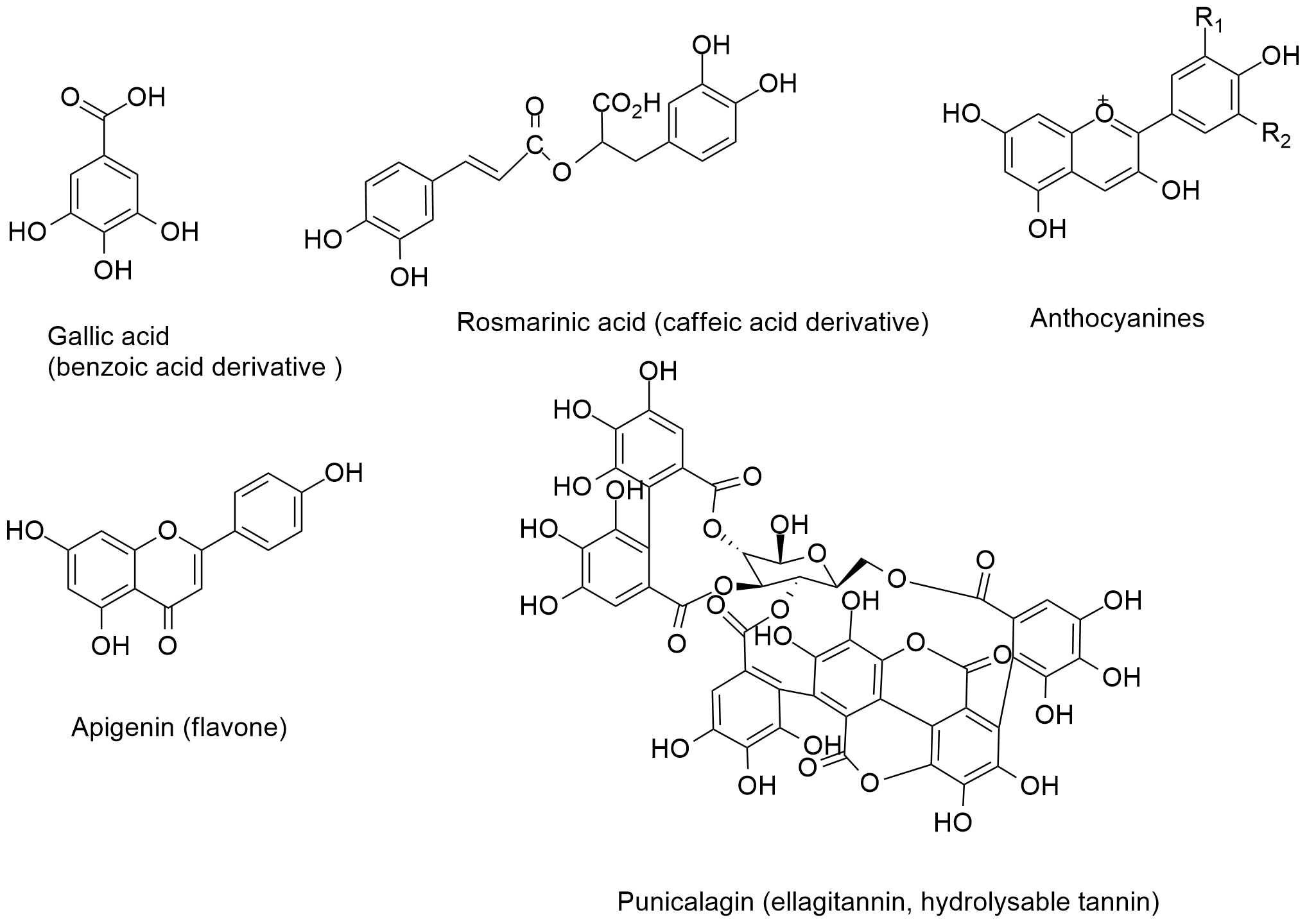
Fig. 3. Representative chemicals structures of major phenolics
